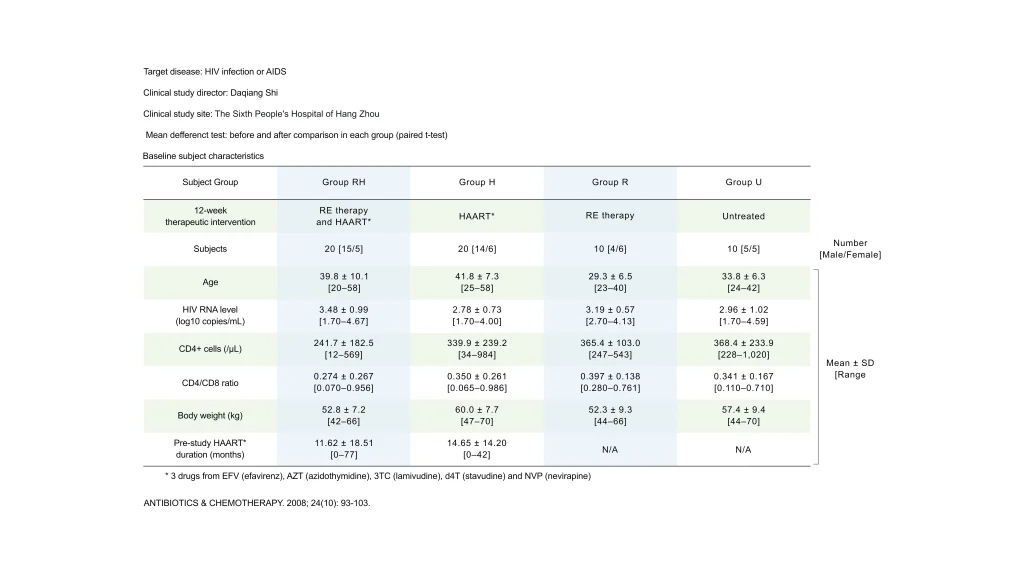
Study Abstract and Baseline Subject Characteristics
As shown in the table below, the four groups underwent each 12-week therapeutic intervention. The mean difference for each group was then compared before and after the intervention.
Outcomes
The Implications of This Clinical Study
The findings of this study showed that the combination of HAART and RE therapy is efficacious in the suppression of HIV viral load, the restoration of cell-mediated immunity, and the enhancement of body weight. Moreover, RE therapy alone exhibited effectiveness in the suppression of HIV viral load and the improvement of body weight.
The effectiveness of HAART, the current standard of care for HIV infection and AIDS, has been established. However, patients must continue to take anti-HIV drugs for the rest of their lives. Therefore, side effects and drug resistance in HIV can sometimes interfere with HAART. The economic cost to patients and society is also a problem.
RE therapy has the following features compared toHAART.
- RE therapy has not been reported to have any serious side effects, and no side effects were found in this study either.
- RE therapy does not directly inhibit HIV replication, in contrast to anti-HIV drugs, so it is unlikely that HIV will acquire resistance to RE therapy.
- Multiple patients can share the use of a single RE therapeutic device.
- RE therapeutic device is a medical device, and unlike anti-HIV drugs, it does not disappear after use.
From the above, the following can be expected of RE therapy.
- Decreasing the dose of anti-HIV drugs, simplifying HAART regimens
- Decrease in the incidence of side effects associated with HAART and the acquisition of HIV drug resistance.
